
Conair, Davis Standard, Zumbach, Leistritz partner for catheter coextrusion application
Canadian Plastics
Plastics ProcessesIn a demonstration of how active pharmaceutical ingredients can be incorporated in a thin layer on the outside of a plastic tube, auxiliary equipment maker Conair Group is teaming up with Davis-Standard LLC and Zumbach Electronics to coextrude a TPU catheter tube at the MD&M West 2018 trade show.
In a demonstration of how active pharmaceutical ingredients (APIs) can be incorporated in a thin layer on the outside of a plastic tube, auxiliary equipment maker Conair Group is teaming up with Davis-Standard LLC and Zumbach Electronics to coextrude a thermoplastic polyurethane (TPU) catheter tube at the MD&M West 2018 trade show.
Typical applications could include the addition of an antimicrobial outer layer to prevent formation of biofilm that can cause infection, Conair said, or an anticoagulant to avoid catheter thrombosis.
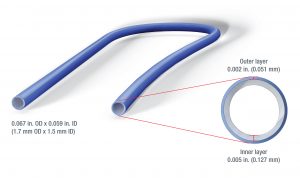
“The same technology that can add color to the surface layer of medical tubing also can be used to apply a pharmaceutically active ingredient like silver sulfadiazine,” said Bob Bessemer, sales manager, medical extrusion for Conair, which is headquartered in Cranberry Township, Pa. “For the demonstration, we are using color instead of an API, but the process is exactly the same.”
The main body of the catheter tube – a single-lumen adult PICC line with an outside diameter of just 0.067 inch – is made of clear TPU delivered by the primary 3/4-inch Davis-Standard extruder. A second 3/4-inch extruder delivers the blue resin representing the active layer. The inner/outer layer ratio is approximately 80/20. Each extruder feeds a positive displacement melt pump, which controls polymer flow to a cross-head co-extrusion die supplied by Guill Tool & Engineering.
“When the line includes single-screw extruders, the API would need to be pre-compounded into the resin or delivered as a masterbatch,” Bessemer said. “But if you use a twin-screw extruder for the pharmaceutical-layer material, you can actually combine the neat API into the polymer in-line with the rest of the process.” American Leistritz, which manufactures twin-screw extruders, is also participating in the demonstration.
As the two-layer tube exits the die, it enters a Conair MedVac vacuum-sizing/cooling tank. The vacuum creates pressure inside that tube that helps stabilize dimensions and prevent cooling water drooling out of the tank’s feed opening from affecting the critical surface finish of the tube. An ultrasonic gauge inside the tank monitors the wall thickness and the thickness of each of the two layers (to 0.0001 inch resolution), while a Zumbach Electronics 3-axis OD laser gauge, positioned downstream from the tank provides not only closed-loop dimensional control, but also displays the tube for concentricity adjustments. The data from these gauges is used to control melt-pump output, as well as puller speed and vacuum to maintain layer thicknesses and other critical dimensions. A vision system is also incorporated into the line to detect imperfections, including color inconsistencies, gels, surface scratches and more. The system communicates in real time with quality controls to automatically separate good vs bad tubes in-line.
Next, the tubing passes through a Conair MedLine puller/cutter. The puller portion of the unit consistently pulls the tubing down the line during sizing and cooling. The rotary-knife cutter slices the tube precisely to the specified length. Cut lengths are then carried away on a Conair medical takeaway belt conveyer. Once on the conveyor, cut pieces that don’t meet quality standards – based on the OD/ID gauge readings and the inline machine-vision – can be removed from the production stream with a puff of compressed air.
MD&M West is being held at the Anaheim Convention Center, in Anaheim, Calif., from February 6 – 8.
