
3D-printed connectors help COVID-19 patients breathe while waiting for ventilators
Canadian Plastics
3D Printing COVID-19The device was created by Belgium-based 3D-printing technology company Materialise, and is now being fast-tracked through the regulatory approval process in Europe and the U.S.
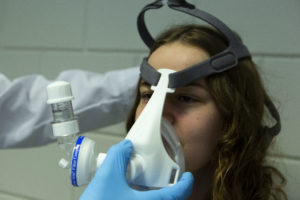
The Materialise Passive NIP is currently being fast tracked through the regulatory process
to make it available as soon as possible during the COVID-19 crisis. Photo Credit: Materialise
As COVID-19 infections continue to rapidly spread, hospitals around the world are in dire need of the necessary medical equipment to treat patients.
Mechanical ventilators are one of these essential items needed during this pandemic which are critically under-supplied. And while many parties are increasing the production of these devices,
Belgium-based 3D-printing technology company Materialise looked at the problem from a different angle. “We saw that clinicians are looking for other ways to deliver positive end expiratory pressure, also known as PEEP, to COVID-19 patients, and worked closely with them for an alternative,” said Brigitte De Vet, vice president of Materialise Medical. “We developed a solution to deliver oxygen and create high positive pressure without the use of a ventilator by designing a 3D-printed connector that holds together standard medical equipment, namely a non-invasive mask, a filter, and a PEEP valve.”
This 3D-printed solution for an adjustable PEEP ventilation is called the Materialise Passive NIP, with NIP standing for non-invasive PEEP. The device converts standard hospital equipment into a non-invasive PEEP mask that can be connected to an oxygen supply. This provides patients with some breathing room before mechanical ventilators are required for treatment. It also helps transition them off ventilators earlier, freeing up these devices for patients in critical need. By using standard medical equipment, including a non-invasive ventilation (NIV) mask, filter and PEEP valve, the solution is simple to use and familiar to medical professionals.
Materialise is now fast-tracking the device through the regulatory approval process in Europe and the U.S. and, in parallel, supporting a clinical trial to test the device’s impact on COVID-19 patients. It expects first results to be available within the next two weeks. “Because the solution was created using 3D design, it makes it possible to manufacture locally and, therefore, bring to hospitals quickly,” De Vet said. “This becomes even more crucial as travel and transport become more difficult. In this case, the product was designed in Belgium and can be printed at a Materialise-certified facility, or at a hospital that has the capability to do so in a reliable manner. Also, smaller production series sizes enabled by 3D printing allow designs to be customized to fit to all types of NIV masks available at hospitals.”
